Our Services
We offer a comprehensive range of services for medical device engineering, development and regulatory:
Concept Development
Our team of experts can help you develop your idea into a viable concept that meets your target market's needs. We can provide market research, competitive analysis, and feasibility studies to ensure your vision is feasible and commercially viable.

Feasibility studies
Feasibility studies are a critical part of the medical device development process. They help to assess the viability of a new product concept and identify any potential challenges or risks. Our feasibility studies include:
- Market research to assess the potential demand for your product
- Technical analysis to determine the feasibility of your product concept
- Regulatory analysis to identify the regulatory requirements
- Cost analysis to determine the cost of developing and manufacturing your product
- Risk analysis for early identification of any risks or challenges associated with your product

Design and Development
We offer full-service design engineering, including CAD modelling, simulation, and analysis. Our engineers are experienced in a wide range of materials and technologies, ensuring we can create the optimal solution for your needs. Risk management is at the forefront of our process, ensuring risks are designed out of the product at the earliest stages. We can help you design a product that is safe, effective, and easy to use. We can also help you optimise the design for manufacturing and ensure that the product is made to the highest quality standards.

Rapid Prototyping
We specialise in rapid prototyping, providing quick turnaround times and iterative design improvements. We have in-house technologies such as 3D printing, vacuum casting, and CNC machining to produce high-quality prototypes that accurately represent your final product. Quick, well-documented, iterative design enables us to bring the best possible device to market in the shortest time possible, also reducing cost by proving the design prior to costly hard tooling.
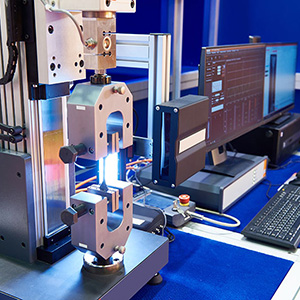
Testing and Validation
Our in-house testing facilities are equipped with the latest equipment and technology to test medical devices for safety and performance, enabling us to prove designs in the shortest time-scales.

Regulatory Compliance
We have extensive experience guiding clients through the complex regulatory landscape of the medical device industry. Our team will help you clearly understand the specific requirements relevant to your product, prepare high-quality documentation, and smoothly navigate the regulatory submission process from start to finish.

Quality Management Systems
We can help you to develop and implement a QMS that meets your company's specific needs and requirements. We can also help you to maintain and improve your QMS over time.

Technical File Creation & Management
Throughout each stage of the project, our team of experts will originate and assemble all documentation required for the device’s technical file, ensuring the technical file is complete, accurate and compliant.

Pre-Production
We provide end-to-end support for pre-production, including tooling design, process development, and validation. Our team ensures your product is manufactured to the highest quality standards and meets all regulatory requirements. We can help you develop the manufacturing process for your product. We can also help you validate the manufacturing process to ensure that it is producing products that meet the required quality standards.
